1000 followers milestone!
May 20, 2020
📣 I am now a Qualified Member of ITI (MITI)!
June 3, 2021MDR-Compliant Italian Translations
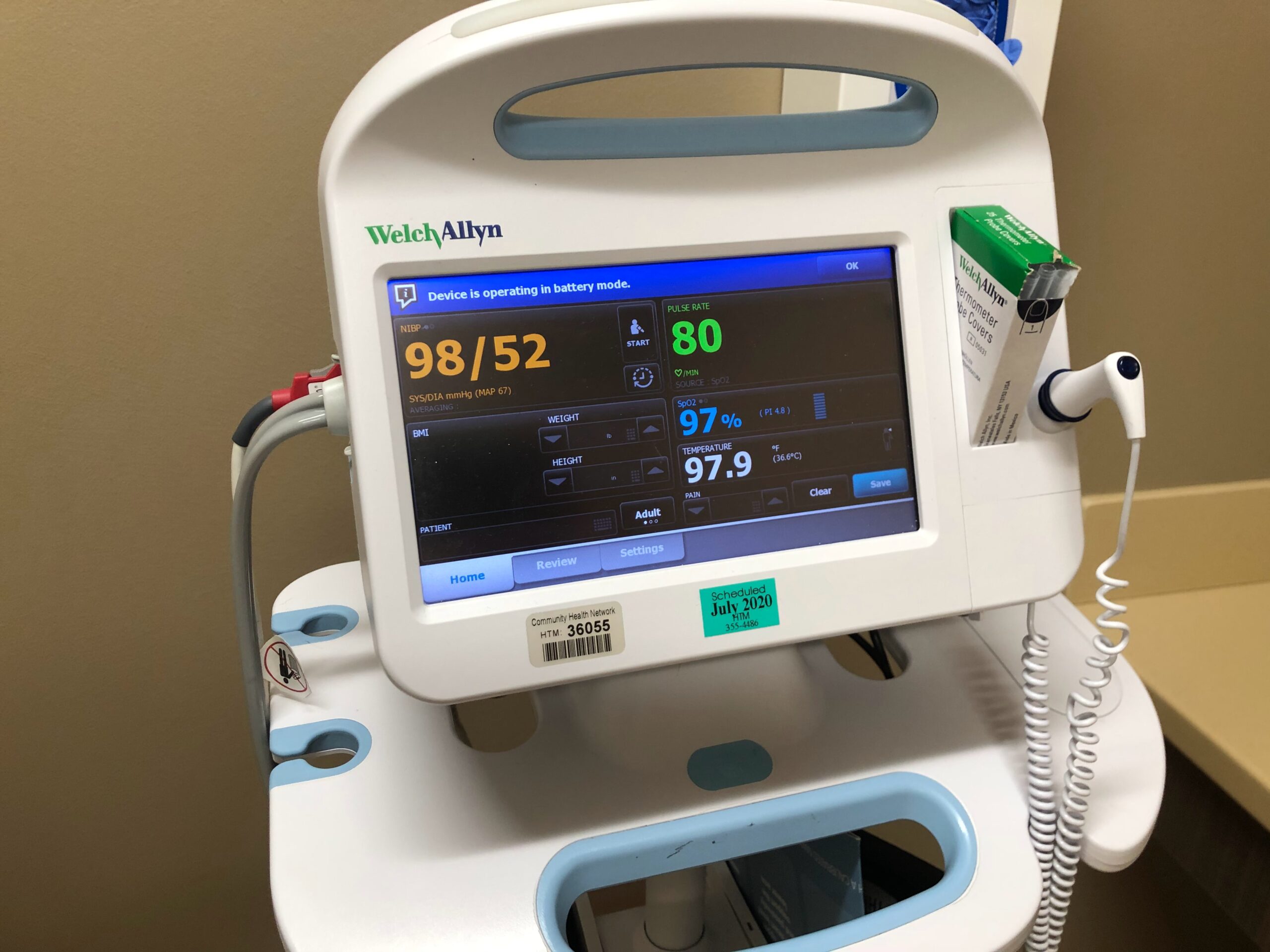
According to the new EU Regulation on Medical Devices (MDR), it is up to distributors/importers to “ensure that the translation of information is accurate and up-to-date”
With the new EU Regulation concerning Medical Devices (EU 2017/745, MDR) coming into force on May 26th 2021, the translated content for Instructions for Use (IFUs) and Product Labeling is part of the Technical Documentation for medical devices. And translation requirements for currently regulated devices have been expanded greatly. Examples of deliverables that will require translation under the MDR Regulation include:
● Labeling development process (Article 10, 11)
● Finalized EU Declaration of Conformity
● Packaging and instructions available for Distributors
● Packaging and instructions published on the Manufacturer's website, and so on.
As you know, the new EU Regulation requires very precise, non-ambiguous, and clear language. IFUs, labeling, safety and clinical performance data must be clear and easy to understand by the intended user, and as a distributor/importer it is up to you to “ensure that the translation of information is accurate and up-to-date”. Therefore, you have to rely on trusted language professionals to translate your documents. Enter, Translating Health. A team of highly skilled Italian professionals with over 100 years' combined experience who can offer you a ready-to-use translation of MDR-compliant IFUs for your medical devices, Periodic Safety Update Reports (PSURs) and Post-Market Clinical Follow-up (PMCFs) reports, catalogs, marketing collateral, and all the required documentation for clinical investigations on/with medical devices. Additionally, we can also localize your medical software. Our approach to translation blends technical and regulatory knowledge, a keen eye for terminology, experience, and critical thinking. All these elements are applied to ensure that your translated materials are accurate and ready to use – either for the launch of a new medical device or for publication. Our job is to make your job easier, and we are good at it.
● Labeling development process (Article 10, 11)
● Finalized EU Declaration of Conformity
● Packaging and instructions available for Distributors
● Packaging and instructions published on the Manufacturer's website, and so on.
As you know, the new EU Regulation requires very precise, non-ambiguous, and clear language. IFUs, labeling, safety and clinical performance data must be clear and easy to understand by the intended user, and as a distributor/importer it is up to you to “ensure that the translation of information is accurate and up-to-date”. Therefore, you have to rely on trusted language professionals to translate your documents. Enter, Translating Health. A team of highly skilled Italian professionals with over 100 years' combined experience who can offer you a ready-to-use translation of MDR-compliant IFUs for your medical devices, Periodic Safety Update Reports (PSURs) and Post-Market Clinical Follow-up (PMCFs) reports, catalogs, marketing collateral, and all the required documentation for clinical investigations on/with medical devices. Additionally, we can also localize your medical software. Our approach to translation blends technical and regulatory knowledge, a keen eye for terminology, experience, and critical thinking. All these elements are applied to ensure that your translated materials are accurate and ready to use – either for the launch of a new medical device or for publication. Our job is to make your job easier, and we are good at it.